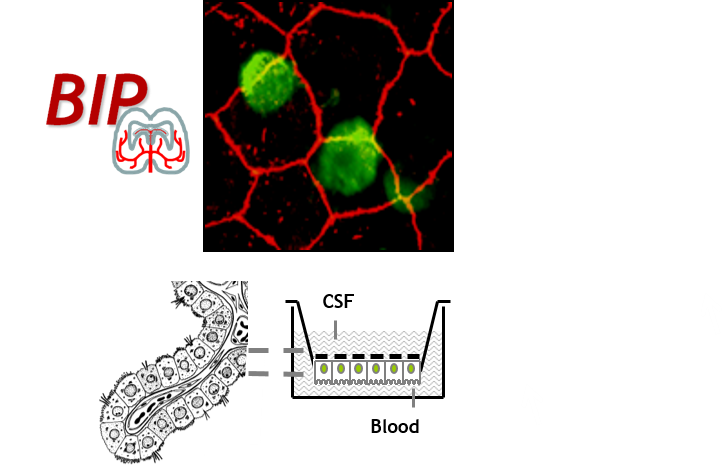
The BIP core facility develops technological tools to:
- study functions of the blood-brain barriers and evaluate neuroprotective strategies restoring barrier efficacy
- evaluate and decipher the mechanisms controlling the cerebral bioavailability of drugs
- investigate immune cell trafficking and pathogen invasion into the brain
The BIP core facility provides counsulting and various services:
- in vivo blood-brain and blood-CSF permeability measurements in developing rodent,
- ex vivo blood-brain interfaces (cerebral microvessels and choroid plexuses) isolation for molecular or functional studies
- use of differentiated cellular models of blood-brain interfaces for molecular transport and cellular migration studies.
Understanding blood-brain interface functions is a key issue to improve CNS drug bioavailability, decrease the side-effects of other non CNS drugs and validate therapeutic targets in these interfaces.
Funded by IHU-CESAME, PIA, “Investment for the Future Program”, the core facility BIP develops relevant tools and provides services to explore the functions (and dysfunctions) of blood-brain interfaces.
BIP also builds up a collection of blood-brain interface tissues from rodents and non-human primates for molecular and biochemical analyses, or immunochemistry.
BIP is open to internal teams (CRNL) and external collaborations (academic research labs, biotech and pharmaceutical companies). Services range from fee-for-service to collaborative research contracts. The facility is equipped and has analytical expertise for spectrophotometry, spectrofluorimetry, fluorescence micro- and macroscopy, protein extraction, UV- Fluo-HPLC, radiodetection, RNA isolation and qRT-PCR, Elisa.
Further information can be obtained by downloading this document.
jean-francois.ghersi-egea@inserm.fr
ns.brain.i@gmail.com